Cellular Plasticity during Liver Regeneration
Regeneration of complex organs depends on diverse cellular mechanisms to restore tissue integrity and function. Broadly, three mechanisms drive this process:
- Proliferation of resident stem cells, such as in skin, intestine or blood.
- Self-duplication of spared functional cells, such as in heart regeneration or mild injury to pancreatic β-cells or liver hepatocytes.
- Transdifferentiation, where one functional cell type converts into another, as seen in severe injuries to bone, pancreatic β-cells, or liver hepatocytes.
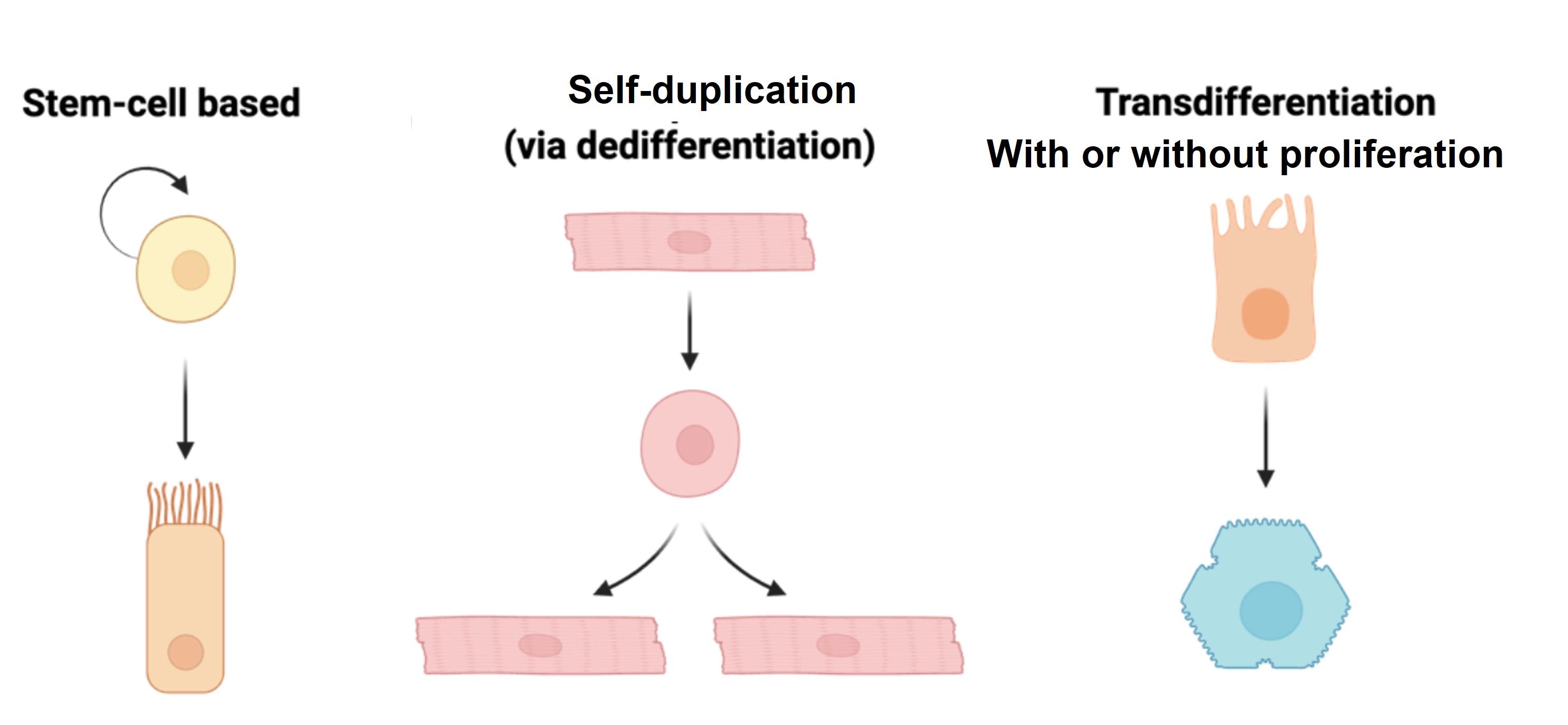
Among these, transdifferentiation is particularly intriguing because it is not the default mode of regeneration. For example, mild injuries to the liver are repaired through self-duplication of existing cells, but in cases of severe injury, where primary sources of regeneration are compromised, transdifferentiation becomes critical. This facultative process allows cholangiocytes (also called biliary epithelial cells) to convert into hepatocytes, compensating for the loss of liver function. Our research focuses on unraveling the molecular and physiological regulators of regenerative plasticity, particularly transdifferentiation, to understand its role in liver repair.
Our lab has uncovered a novel and unexpected role for cholangiocyte plasticity during rapid developmental growth, where transdifferentiation occurs not in response to pathology but as a physiological mechanism to meet the demands of rapid organ expansion. This finding challenges the prevailing paradigm that transdifferentiation is purely a compensatory mechanism and highlights its critical role in normal developmental processes. Read the pre-print here
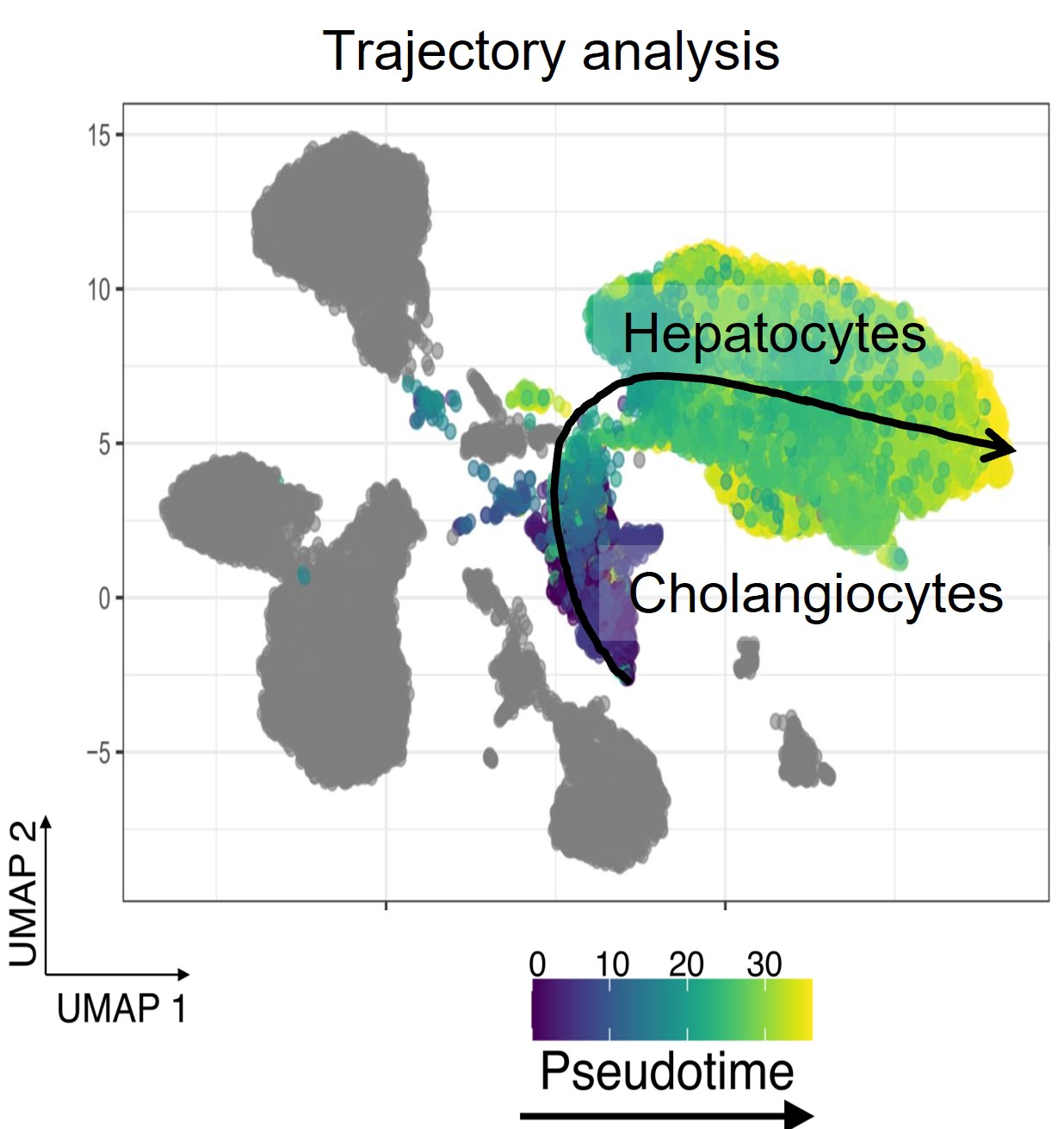
Unlike in adults, where hepatocyte senescence often serves as a trigger for cholangiocyte-driven regeneration, our work shows that during growth spurts in zebrafish, cholangiocyte-to-hepatocyte conversion occurs independently of hepatocyte senescence. This unique context provides an exceptional opportunity to study the molecular drivers and physiological triggers of transdifferentiation in a non-pathological setting.
Our research focuses on identifying the regulators that enable this remarkable plasticity and understanding how developmental growth environments influence cellular behavior. By leveraging advanced genetic tools, live imaging, and transcriptomic analyses in zebrafish, we aim to uncover the fundamental principles governing BEC-to-hepatocyte conversion. These insights have broader implications, offering a blueprint for exploring regenerative mechanisms in both health and disease contexts, and paving the way for advancing our understanding of organ repair across species.