Response to starvation stress
Starvation is a severe form of malnutrition that occurs when an individual’s intake of food is inadequate to meet their body’s energy requirements. Prolonged starvation can cause permanent organ damage, stunted growth in children, and eventually death if left untreated. It is estimated that approximately 10% of the global population suffered from chronic undernourishment in 2021, and that approximately 45% of deaths among children under the age of 5 years are linked to undernutrition. This figure has been steadily increasing in recent years, partly due to factors such as conflicts and climate change. Developing interventions aimed at improving starvation resistance is critically needed to fight against nutritional deficiency. Surprisingly, despite the widespread prevalence of starvation, there has been considerably more research focusing on preventing tissue damage resulting from overconsumption than from prolonged hunger.
Notably, the liver’s function and health are compromised during starvation. Almost 90 years ago, the pediatrician Dr. Cicely Williams recognized that chronic severe malnutrition in children leads to fatty liver, and named the condition as ‘kwashiorkor’. Subsequently, starvation has been shown to induce accumulation of liver fat (steatosis) in drosophila, zebrafish, and mammals, including mice, minks, cats & humans. Notably, cats suffering from sudden anorexia, due to decreased food availability or secondary to a disease, develop feline hepatic lipidosis (FHL), which is the most common hepatobiliary disease in cats. The prognosis from FHL can be positive but only with appropriate and fast nutritional treatment as mortality can be close to 100% without aggressive nutrition therapy. Pharmacological interventions to protect the liver during starvation are non-existent.
Additionally, a major driver of evolutionary selection is the adaptation to starvation as animals in the wild face uncertain food supply. Adaptation to periods of famine shape physiology in a variety of species: fatty liver in migratory birds, high blood sugar in seals and insulin resistance (IR) in hibernating bears.
Our work has demonstrated that the zebrafish liver accumulates lipid droplets in response to starvation. Hepatic steatosis, in this case, creates an energy reservoir that allows survival during long-term caloric deprivation. We study this “natural” mode of liver steatosis to uncover the mechanisms underlying its induction and resolution, identifying insulin-driven calcium signaling and a lipid transporter (slc27a2 / FATP2) as a regulator of fatty liver. As steatosis is the first step towards liver disease, we are currently investigating the link between steatosis and liver damage.
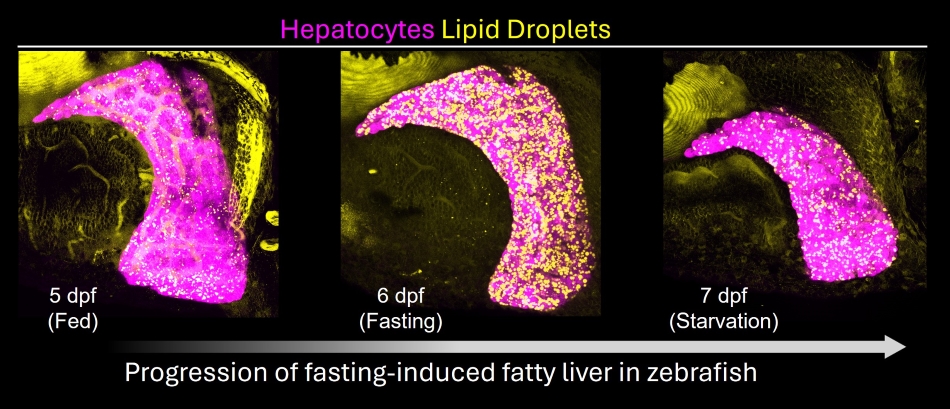
Development of fatty liver in fasting zebrafish larvae.
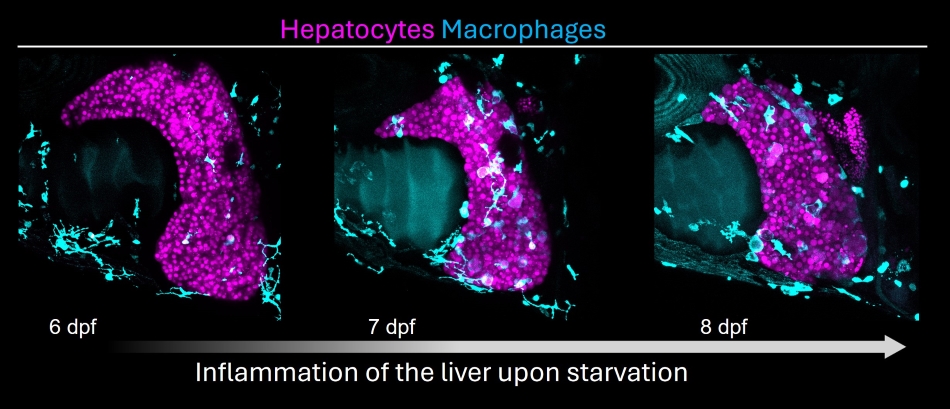
Inflammation (macrophages) in the starved liver.